
The key challenges outlined in this assessment relate to the production delays in ramping up the required production volumes and in the “final mile” challenges in distribution.
With President Biden’s new administration, it is expected that the US government will take a much more hands on and ambitious approach in tackling the Covid-19 vaccination distribution challenge.
This once in a lifetime event now needs to be met heads on. Not just with a clear ambition but with an even greater sense of urgency to execute and deliver on getting vaccines produced and distributed to people around the world quickly. 
Production Challenges
So what are the key challenges? First, annualized production capacity based on the production output figures announced by the vaccine manufacturers seems well short to deliver vaccines to the whole world in 2021.
Based on the above numbers, the current annualized production capacity comes to 9.3 Billion vaccines. This is annualized production capacity much of which still needs to be turned on in the first half of 2021. Based on the production lag in ramping up to these volumes, experts predict less than 20% of the world’s population can be vaccinated in 2021 at the current rate of production.
Second, production output across all manufacturers is way behind what was promised earlier. In 2020, we were told that vaccine manufacturers were producing vaccines at risk whilst finalizing the clinical trial studies and awaiting approval for their vaccines from the regulatory authorities.
Vaccines is big business. The EU and some other governments this week have suggested suing companies like AstraZeneca over delays and shortages in delivery. Pfizer has reported a short-term drop in production. Others have simply not yet been able to ramp up commercial production capacity. 
Many pharmaceutical companies rely on external capacity at CMO’s to beef up their throughput. Much of the fill and finish production capacity is outsourced. The vaccine manufacturing process is a delicate and complicated process involving a number of manufacturing steps often across various production partners and facilities.
In recent months, many manufacturers have had to reach out to sub-contract or partner with other pharmaceutical companies to scale up and create additional production capacity.
The new US administration has already indicated it will deploy, if needed, the US Defense production bill from 1951 to ensure that greater production capacity is made available to fight this crisis. This sends a clear signal that more production capacity is needed and that manufacturers should work harder and faster together to produce more vaccines from more facilities around the world.
An example this week of collaboration suddenly coming off the ground is between Sanofi and Pfizer. Sanofi announced in a surprise move that it will make production capacity available to be able to start producing Pfizer vaccines by July 2021. This would increase annualized production by a further 100 million vaccines. 
Distribution Challenges
The second set of challenges centers around the distribution of the vaccines. In December 2020, pictures of courier deliveries led us believe that the vaccines were being rolled out quickly at least across some parts of the world.
The sad reality is that the distribution process has not delivered what it should have. President Biden called the coronavirus vaccine rollout effort on January 20 “a dismal failure so far”. In most other countries, government vaccine programs have also seen a slow and rocky start.
There lies the issue, the biggest bottleneck is in the last mile in getting the vaccines to the actual points of dispensing. This is currently a weak spot in the whole distribution model which needs to be sorted out as soon as possible.
Vaccine manufacturers will probably argue that the “last mile” is a responsibility for governments to organize as part of their own domestic immunization strategy. However, in the regular commercial model of pharmaceutical distribution, the dispensing of medicines and drugs to hospitals, pharmacies and other points of dispensing is typically organized by the manufacturers. 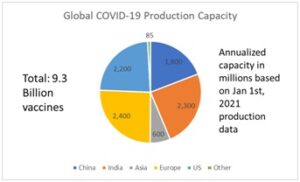
They also manage the forecasting, the replenishment models and have the tools and technologies in place to manage supply planning more effectively through their supply chain organization and logistics partners.
Governments traditionally do not have the knowledge nor the people in place in organizing the supply chain for pharmaceuticals. Given the enormous scale of this pandemic, most governments simply do not have the required expertise to manage any of this. It’s therefore imperative for governments and manufacturers alike to accept that the final mile bottlenecks need to be fixed and that they need professional supply chain support to do so quickly. 
Recommendations
It’s now critical to learn from the mistakes made, put possible egos aside and to adjust existing distribution models quickly. Typically, pharmaceutical companies utilize supply chain consultancy expertise to set up their initial distribution model of a new medicine. In fact, the distribution model of about 80% of all new drugs launched by pharmaceutical companies are designed with the support of specialized pharmaceutical supply chain consultants and not by logistics service providers or courier companies.
Integrating the final mile into the distribution model will require greater collaboration between manufacturers and governments. Although each country and government will face unique distribution challenges there are a set of standard universal supply chain principles which require more thought. One element is building more intelligence into the supply chain using data and demand planning tools to ensure there is more information to plan and manage the end-to-end inventory points and the whole replenishment model.
Another key aspect is thinking more carefully about points of dispensing to ensure more vaccines reach people more quickly. Planning the whole supply chain more holistically to ensure that not only the vaccines arrive on time but also the other medical supplies needed such as needles, syringes, the dilution liquids, as well as the medical staff required to conduct the actual vaccinations. This too requires smarter planning solutions.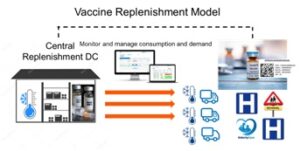
Technology and use of big data should play a far bigger role in the management of the whole distribution and replenishment model related to the COVID-19 vaccines.
The pharmaceutical industry in recent years has invested heavily in barcoding technology to improve visibility and traceability at item level. One key enabler would be in making greater use of this technology. It would also enhance security around the supply of vaccines.
This barcode technology can easily be used to better track products at item level, including the consumption levels at place of dispensing and therefore help to better manage the whole distribution process more dynamically. This will help manufacturers and governments with improved visibility and insight on what is consumed per dispensing point to be able to better organize the replenishment model based on real demand and inventory data. Having a centralized logistics control room manage such a replenishment model is imperative to the whole solution. 
There needs to be recognition that the COVID-19 distribution challenge will not go away by itself and will not be resolved with well intentioned but half-baked measures.
Demand, supply, and inventory must be managed more holistically and more intelligently. It cannot be that the supply chain for this critical global vaccination program continues to be fragmented and disjointed. This is a massive multi-level global, national, and local distribution exercise never seen before on such a scale.
Europhia Consulting is an international management consulting company specialized in the logistics and supply chain industry in the life sciences sector. We operate global assignments for our clients. The opinions are based on the author’s own experience and understanding of the dynamics within the sector.
Eelco Dijkstra of Europhia Consulting has worked in supply chain and consultancy for over 25 years and in recent years has focused his expertise on the global pharmaceutical sector.
Email: europhia@outlook.com, Website: www.europhia.com
Biolog Consulting is specialized in Life Science & Healthcare Supply Chain consulting. From strategy to operations, we bring our expertise of hundreds of successful missions to our clients to make them deliver the right product at the right time to the right patient. The company operates globally and uses successful methods, with a pragmatic approach to project management, which always respond to client needs.
Franck Toussaint of Biolog Consulting has extensive experience in the life sciences industry in Belgium and internationally. Successful manager and supply chain management expert with a large experience in driving performance and change across organizations.
Website: www.biolog-consulting.com, Email: fto@biolog-consulting.com
Supply Chain Operations SA, based in Switzerland, is a specialized healthcare supply chain consultancy firm created in 2011 to serve the bio-pharmaceutical and medtech industry. We bring more than 120 years of end-to-end supply chain expertise to our valued customers.
Laurent Foetisch of Supply Chain Operations has extensive experience as a supply chain executive responsible for managing a global bio- pharmaceutical company in Switzerland for more than 20 years. In 2007 Laurent managed the supply chain integration between Merck and Serono with the responsibility to create one central supply chain structure using common processes and tools. Since 2011, Laurent runs his own supply chain boutique consulting firm named Supply Chain Operations.
Email: laurent.foetisch@supplychainoperations.ch, Website: www.supplychainoperations.ch
Europhia Consulting, Biolog Consulting and Supply Chain Operations are at the front end of global supply chain consultancy assignments related to COVID-19 developments since the start of the pandemic in working with governments, manufacturers, and logistics service providers. The companies have written multiple white papers on the topic of COVID-19 distribution. See also: https://europhia.com/covid-19-supply-chain






